Abstract
Background: In myelofibrosis (MF), patients with cytopenias have posed a therapeutic challenge, as the first two approved JAK2 inhibitors exacerbate anemia and thrombocytopenia. In contrast, newer JAK2 inhibitors, have been associated with hematologic stability and, in some cases, anemia benefit. For example, patients treated with the newly approved JAK2/IRAK1 inhibitor pacritinib were more likely to achieve clinical improvement in hemoglobin compared to best available therapy (BAT) in the Phase 3 PERSIST-2 study. This effect may be the result of enhanced erythropoiesis via IRAK1 inhibition by pacritinib. Similarly, it has been postulated that inhibition of activin A receptor, type I (ACVR1), which mediates hepcidin production, is able to overcome the JAK2 inhibitor class effect on anemia and result in improvement in transfusion independence (TI). The impact of pacritinib on TI has not previously been described, nor has a mechanism been elucidated by which pacritinib improves anemia. Here, we show that pacritinib is a potent ACVR1 inhibitor with a clinically important impact on TI in patients with MF.
Methods: Patients treated on PERSIST-2 were included in this analysis provided they were not TI at baseline and had enrolled ³12 weeks prior to study termination. All patients had platelet counts ≤100x109/L as an enrollment criterion. The percentage of patients who became TI was compared between pacritinib 200 mg BID and BAT through week 24. The subgroup of BAT that received erythroid support (erythropoiesis stimulating agents, danazol, thalidomide or analogs, or corticosteroids) was also analyzed. TI was defined using both Gale criteria (absence of RBC transfusions over a 12-week period) and criteria employed in the pivotal studies of momelotinib ("SIMPLIFY criteria": absence of RBC transfusions and no hemoglobin <8 g/dL). The inhibitory activity of pacritinib, momelotinib, fedratinib, and ruxolitinib against ACVR1 was assessed using a HotSpot assay (Reaction Biology Corporation). The half maximal inhibitory concentration (IC50) was calculated using 3-fold serial dilutions starting at 10µM.
Results: Baseline characteristics of patients who were not TI (SIMPLIFY criteria) were similar between pacritinib (n=42) and BAT (n=44), including median hemoglobin (8.7 vs. 8.6 g/dL), median platelet count (43 vs. 41 x109/L), and percentage who received prior JAK2 inhibitor therapy (55% vs. 57%). Among patients with JAK2V617F, most patients in both treatment groups had <50% allele burden (75% [27/36] vs. 74% [26/35]).
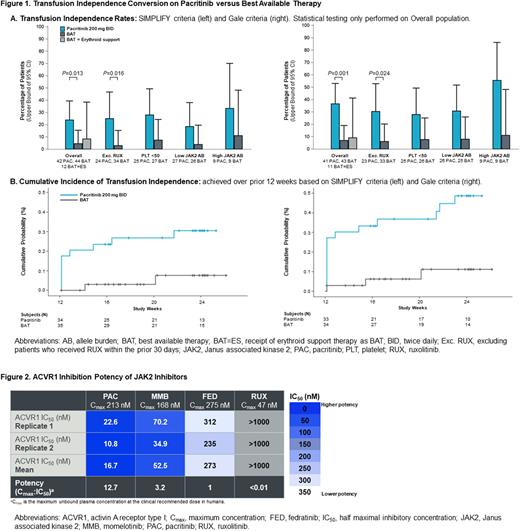
A significantly greater proportion of pacritinib-treated patients achieved TI compared to BAT (24% vs. 5%, P=0.013 based on SIMPLIFY criteria; 37% vs. 7%, P=0.001 based on Gale criteria), as shown in Figure 1A. The TI conversion rate for patients who received erythroid support therapies as BAT (8% with SIMPLIFY criteria; 9% with Gale criteria) was similar to the overall BAT group. The effect size for pacritinib was maintained among patients who had not received ruxolitinib within 30 days prior to treatment initiation, suggesting that conversion to TI was related to a pacritinib treatment effect rather than to a rebound effect of prior ruxolitinib. TI conversion rates were higher on pacritinib compared to BAT in patients with platelet counts <50x109/L and in those with low and with high JAK2V617F allele burden. Pacritinib-treated patients achieved TI throughout the study through week 24 (Figure 1B).
To evaluate possible mechanisms of erythropoietic benefit, we measured ACVR1 inhibition and found that the IC50 of pacritinib against ACVR1 was 16.7 nM, which is 12.7-fold lower than the maximum concentration (Cmax) achieved clinically with pacritinib 200 mg BID. Momelotinib inhibited ACVR1 with a mean IC50 of 52.5 nM, which is 3.2 times lower than the clinical Cmax. Neither fedratinib nor ruxolitinib were potent ACVR1 inhibitors (Figure 2).
Discussion: Pacritinib exhibited four-fold higher potency for ACVR1 compared to momelotinib and is associated with a clinically and statistically significant improvement in transfusion requirement in patients with MF. In contrast, JAK2 inhibitors that do not inhibit ACVR1 tend to worsen anemia. Further studies are needed to establish the mechanisms underlying the benefits of pacritinib in anemic patients, including the role of IRAK1 and ACVR1 inhibition.
Disclosures
Oh:PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees; Disc Medicine: Consultancy, Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Kartos: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgne/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees; Blueprint Medicines: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees. Mesa:AbbVie: Research Funding; Sierra Oncology: Consultancy, Research Funding; Novartis: Consultancy; CTI: Research Funding; Celgene: Research Funding; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Research Funding; Samus: Consultancy, Research Funding; LaJolla Pharmaceutical: Consultancy; AOP: Consultancy; Promedior: Research Funding; Genotech: Research Funding; Incyte: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy; Blueprint: Consultancy; Geron: Consultancy; Roche: Consultancy; Gilead: Research Funding; Imago: Research Funding. Harrison:Galecto: Consultancy, Membership on an entity's Board of Directors or advisory committees; Galacteo: Membership on an entity's Board of Directors or advisory committees; Sierra: Honoraria; Incyte: Speakers Bureau; Celgene/BMS: Membership on an entity's Board of Directors or advisory committees, Research Funding; Geron: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; EHA: Other: Leadership role; Gilead: Membership on an entity's Board of Directors or advisory committees; Promedior: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; MPN voice: Other: Leadership role; Shire: Membership on an entity's Board of Directors or advisory committees; AOP Pharma: Consultancy, Membership on an entity's Board of Directors or advisory committees; Keros: Consultancy; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings, Research Funding. Bose:Kartos: Honoraria, Research Funding; Sierra Oncology: Honoraria; Karyopharm: Honoraria; Ionis: Research Funding; Incyte: Honoraria, Research Funding; CTI BioPharma: Honoraria, Research Funding; PharmaEssentia: Honoraria; Cogent: Honoraria, Research Funding; Blueprint: Honoraria, Research Funding; BMS: Honoraria, Research Funding; AbbVie: Honoraria; Morphosys: Honoraria, Research Funding; NS Pharma: Research Funding; Promedior: Research Funding. Gerds:Kratos Pharmaceuticals: Research Funding; Accurate Pharmaceuticals: Research Funding; Incyte Corporation: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imago BioSciences: Research Funding; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb/Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Sierra Oncology: Consultancy, Membership on an entity's Board of Directors or advisory committees; Morphosys/Constellation: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; PharmaEssentia: Consultancy, Membership on an entity's Board of Directors or advisory committees. Heaney:Blueprint Medicine: Consultancy, Research Funding; Cogent Biosciences: Consultancy, Research Funding; Hoth Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; Kartos: Research Funding; Novartis: Consultancy, Research Funding; PharmaEssentia: Consultancy; Sierra Oncology: Consultancy, Research Funding; BMS: Research Funding; CTI BioPharma: Consultancy, Research Funding. Gupta:AbbVie: Consultancy, Other: Participation on a Data Safety or Advisory board; Roche: Other: Participation on a Data Safety or Advisory board; Sierra Oncology: Consultancy; Pfizer: Consultancy, Other: Participation on a Data Safety or Advisory board; BMS Celgene: Consultancy, Honoraria, Other: Participation on a Data Safety or Advisory board; Novartis: Consultancy, Honoraria; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy, Honoraria. Scott:Nektar: Other: data and safety monitoring board; Jazz Pharmaceuticals: Other: Advisory Panel; Novartis: Other: Advisory Panel, Research Funding; Alexion: Consultancy; Celgene: Consultancy, Honoraria, Other: Advisor Panel; Bristol Myers Squibb: Consultancy, Honoraria, Other: Advisory Panel, Research Funding; Johnson and Johnson: Other: data and safety monitoring board; Incyte: Consultancy. Kiladjian:BMS: Membership on an entity's Board of Directors or advisory committees; AOP Orphan: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees. Lucchesi:SOBI: Speakers Bureau; Pfizer: Speakers Bureau; Incyte: Speakers Bureau; Morphosys: Consultancy; Amgen: Consultancy, Speakers Bureau; Grifols: Consultancy, Speakers Bureau; Novartis: Consultancy, Speakers Bureau; BMS: Speakers Bureau; Sanofi: Consultancy, Speakers Bureau. Buckley:CTI BioPharma: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Tyavanagimatt:CTI BioPharma: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Roman-Torres:CTI BioPharma: Current Employment, Current equity holder in private company, Current holder of stock options in a privately-held company. Mascarenhas:CTI BioPharma: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Merck: Research Funding; PharmaEssentia: Consultancy, Research Funding; Forbius: Research Funding; Celgene/BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy; Constellation Pharmaceuticals, Inc., a MorphoSys Company: Consultancy; AbbVie: Consultancy, Research Funding; Kartos: Consultancy, Research Funding; Geron: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Galecto: Consultancy; Merus: Research Funding; Janseen: Research Funding; Sierra Oncology: Consultancy; Prelude Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Consultancy, Membership on an entity's Board of Directors or advisory committees; Imago: Consultancy; Roche: Consultancy, Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Verstovsek:Novartis: Consultancy, Research Funding; AstraZeneca: Research Funding; Protagonist Therapeutics: Research Funding; Blueprints Medicines Corp.: Research Funding; ItalPharma: Research Funding; Promedior: Research Funding; NS Pharma: Research Funding; PharmaEssentia: Research Funding; Genentech: Research Funding; Gilead: Research Funding; Incyte: Consultancy, Research Funding; Roche: Research Funding; Sierra Oncology: Consultancy, Research Funding; Constellation Pharmaceuticals: Consultancy; CTI BioPharma Corp.: Research Funding; Celgene: Consultancy, Research Funding; Pragmatist: Consultancy.
OffLabel Disclosure:
Pacritinib is a kinase inhibitor indicated for the treatment of adults with intermediate or high-risk primary or secondary (post-polycythemia vera or post-essential thrombocythemia) myelofibrosis with a platelet count below 50 Ãâ€" 10^9/L. This indication is approved under accelerated approval based on spleen volume reduction. Continued approval for this indication may be contingent upon verification and description of clinical benefit in a confirmatory trial(s).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal